Understanding the Factors Affecting the Formation of Carbonyl Iron Electrodes in Rechargeable Alkaline Iron Batteries
Understanding the Factors Affecting the Formation of Carbonyl Iron Electrodes in Rechargeable Alkaline Iron Batteries
Aswin K. Manohar, Chenguang Yang, Souradip Malkhandi, Bo Yang, G. K. Surya Prakash and S. R. Narayanan,z
Abstract
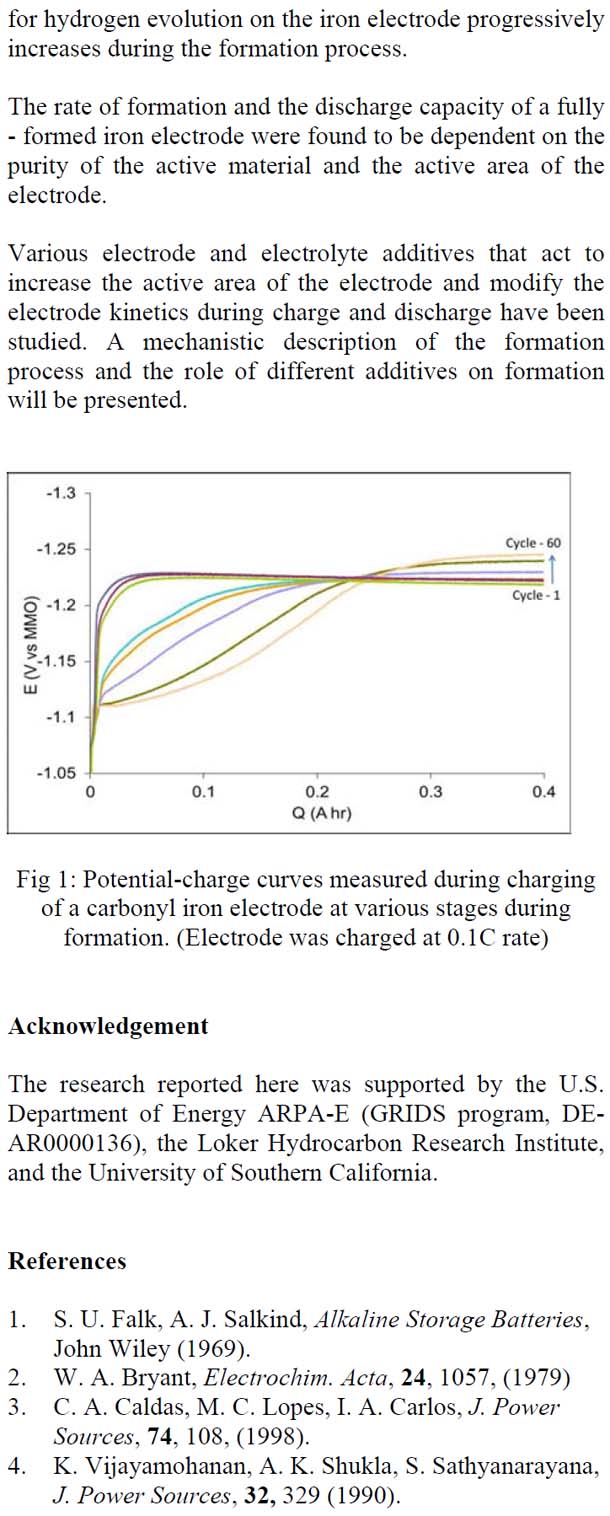
Rechargeable iron-based alkaline batteries such as iron – air and nickel – iron batteries are attractive for large-scale electrical energy storage because iron is inexpensive, globally-abundant and environmentally-friendly. Further, the iron electrode is known for its robustness to repeated charge/discharge cycling. During manufacturing these batteries are charged and discharged 20 to 50 times during which the discharge capacity of the iron electrode increases gradually and attains a stable value. This process of achieving stable capacity is called formation. In this study we have focused our efforts on understanding the effect of electrode design on formation. We have investigated the role of wetting agent, pore-former additive, and sulfide additive on the formation of carbonyl iron electrodes. The wetting agent increased the rate of formation while the pore-former additive increased the final capacity. Sodium sulfide added to the electrolyte worked as a de-passivation agent and increased the final discharge capacity. We have proposed a phenomenological model for the formation process that predicts the rate of formation and final discharge capacity given the design parameters for the electrode. The understanding gained here will be useful in reducing the time lost in formation and in maximizing the utilization of the iron electrode.
Article from: http://jes.ecsdl.org/content/159/12/A2148.abstract
Pdf link: http://ma.ecsdl.org/content/MA2012-02/5/371.full.pdf+html or Here
Read also