CVMR®’s Lithium Metal
Lithium is the lightest solid element with half the density of water. It is highly reactive and prone to spontaneous combustion. Lithium looks like Sodium and Potassium and is of the same alkali metal group.
Metallic Lithium has the highest specific heat capacity of any solid element and is used in the heat transfer applications; however, it is corrosive and requires special handling. The metal is a good alloying agent, used in lithium aluminum and lithium
magnesium alloys in aircrafts, where it imparts a high-temperature strength, improves elasticity, and increases tensile strength. It also has nuclear applications.
Due to its high electrochemical potential, lithium is used as a battery anode material. Glass and ceramic industries are another two sectors that use Lithium. Lithium Chloride and Lithium Bromide are used in air conditioning and industrial drying
systems.
Lithium Occurrence
Lithium is mainly available in brines and hard rock minerals. In Mongolia it can be found encased and fossilized in clay. Volcanic origin brines are present in desert areas and occur in dry lake areas and interior desert basins where Lithium has been
concentrated by solar evaporation.
In dry lake areas the basin surfaces are predominantly composed of silts and clays, which contain Lithium with some salt incrustation. In the interior desert basins, sometimes known as salt lakes or “salars”.
Extracting lithium from brines found below the surface of dried lakebeds (salars) and from mineral deposits has been the dominant method of producing lithium. High grade lithium compounds are processed mostly by solar evaporation of salar brines in
Argentina, Chile, and Bolivia. Lithium is present in very high concentrations in these brines (typically more than 500 milligrams of lithium per liter of brine), and processing costs are low. However, lithium separation from salar brines has several
disadvantages. Separation is slow (taking up to 24 months), weather-dependent, and has an extraction efficiency of only about 50%. After lithium is concentrated by solar evaporation, it still requires multiple purification steps.
CVMR’s process allows the rapid extraction of lithium with a high recovery rate, taking hours instead of months. The product of the process is a high purity grade lithium carbonate for use in manufacturing lithium batteries.
Advantages of CVMR’s Proprietary lithium Processing
• Speed and efficiency of extraction: CVMR®’s process allows for rapid extraction
of lithium with a high recovery rate, taking hours instead of months in the case of extraction from brine;
• Reduced production cost;
• Smaller footprint with less environmental impact;
• The final product is high purity grade lithium carbonate used in the manufacturing
of lithium batteries.
Mining and Refining of Lithium
CVMR® has the capability and extensive experience to manage various aspects of lithium extraction from brine, spodumene ore and clay, and has conducted Lithium refining, from concept to production in Bolivia, Mongolia, and Chile. We have also worked
extensively on the uses of lithium such as lithium-ion cells, batteries, lithium compounds, ceramics, and lithium alloys.
CVMR® can approach a Lithium project in several ways depending on the composition and mineralogy of the reserve. The choice of method used in refining lithium is usually made upon examination and a series of tests to determine the amenability of the
lithium bearing brine to one or the other proprietary methods used by CVMR®.
CVMR®’s Experience in Lithium Extraction and Refining
CVMR® has designed extraction and refining processes of lithium for Ironstone Resources of Alberta, Canada, the Uyuni Salt Flats of Bolivia, and lithium brine fossilized ore for Alkalli Metals and for the government of Mongolia.



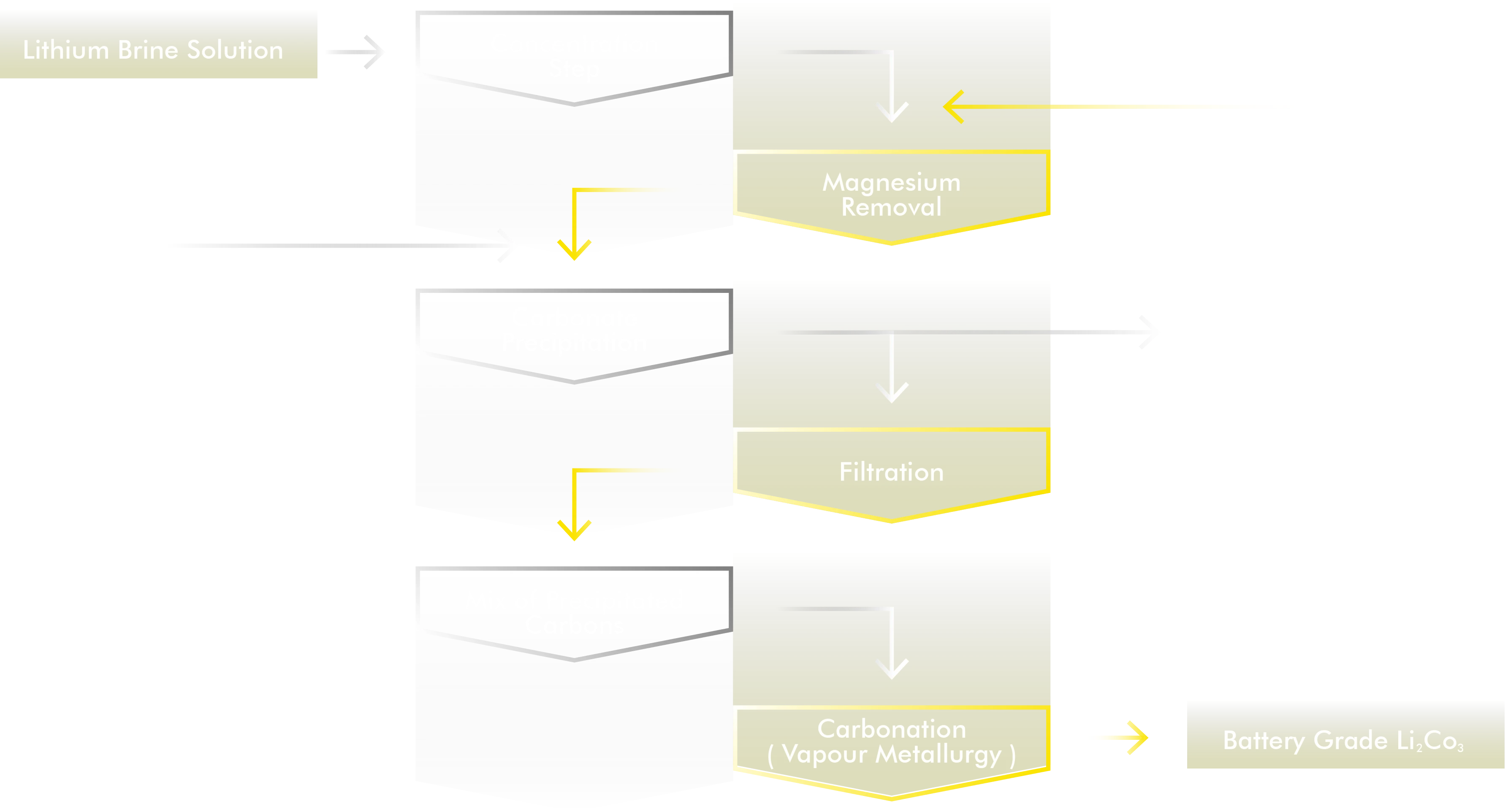
Extraction and Refining lithium from brine with high magnesium content in Bolivia
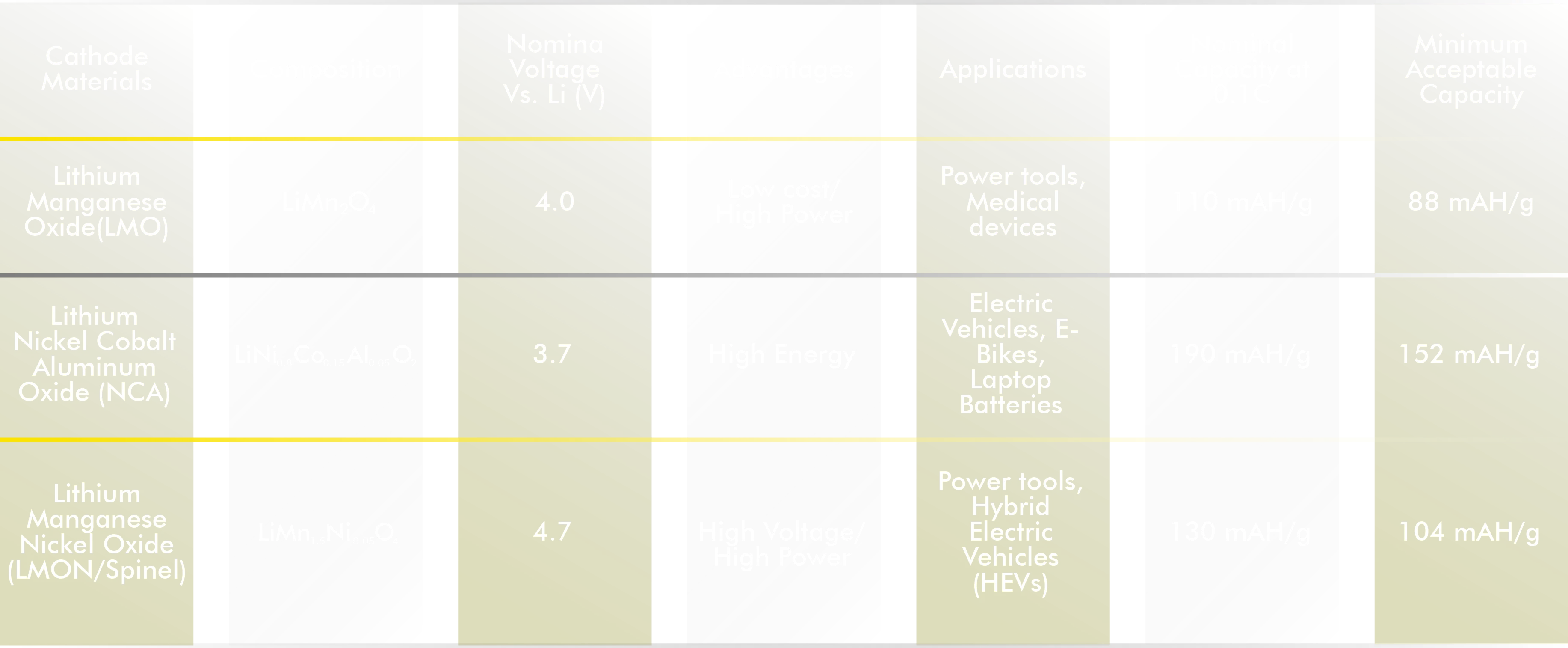
Varieties of Lithium-Ion Batteries
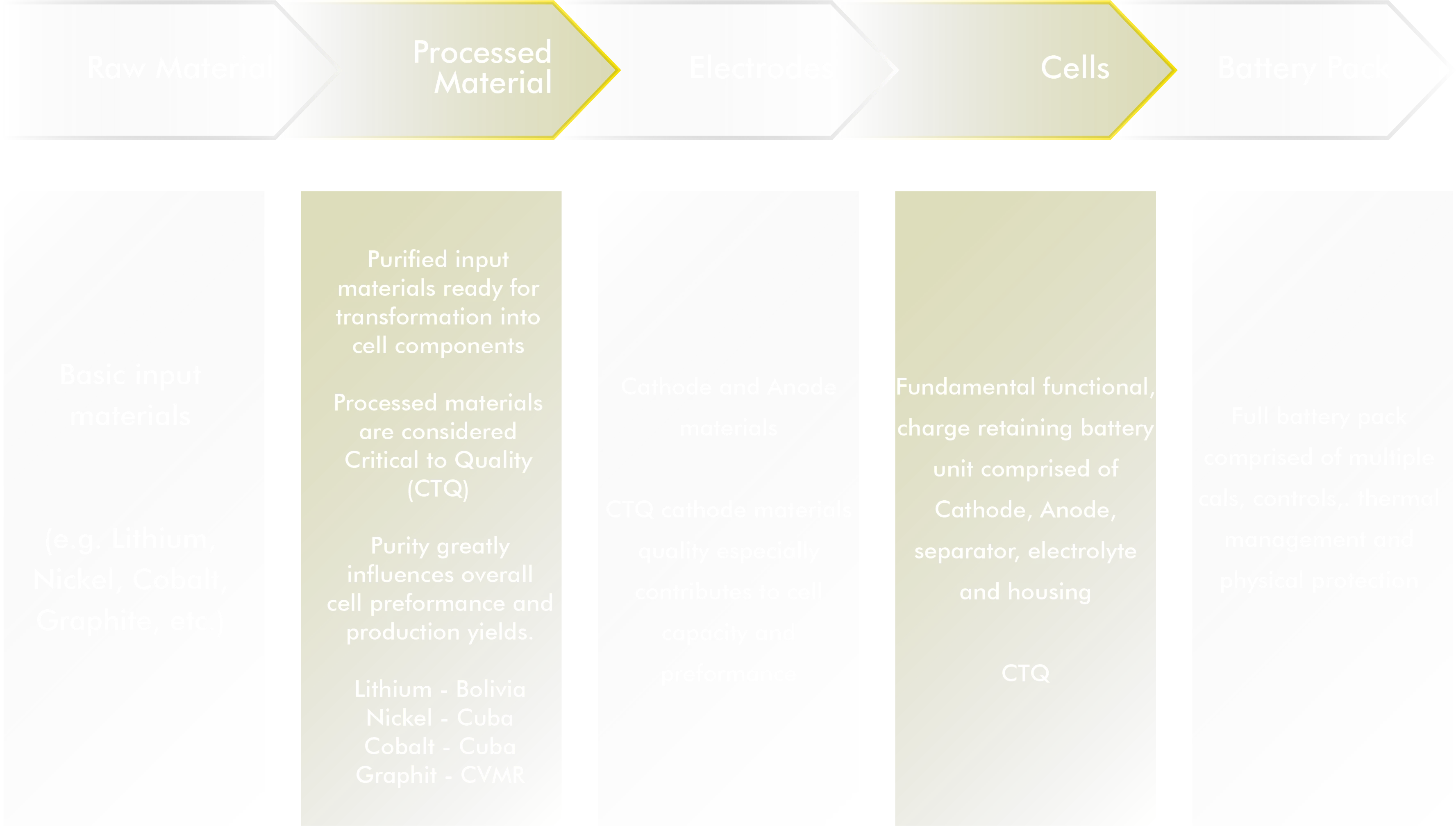
Lithium-Ion Battery Manufacturing Value Chain