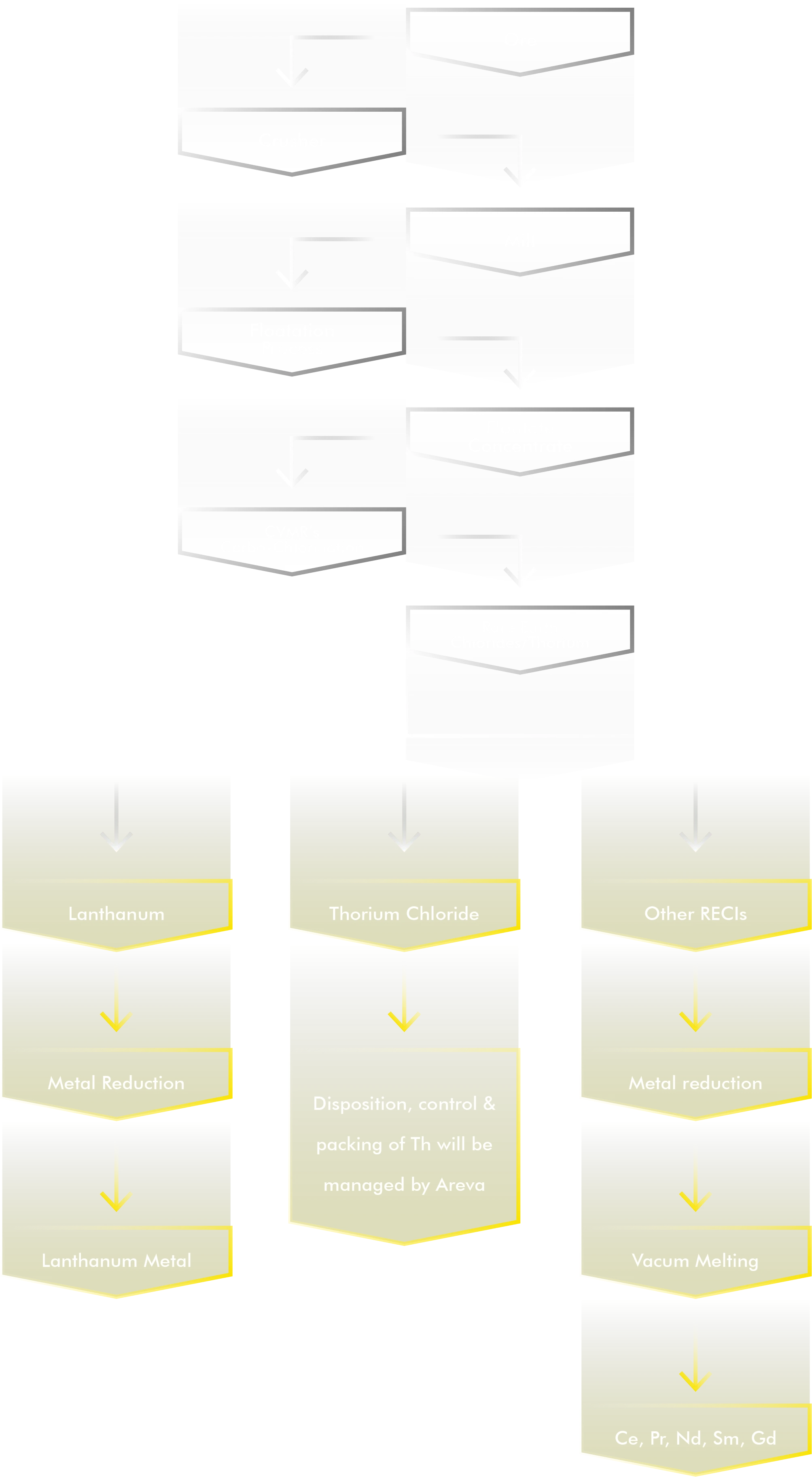
CVMR®’s Rare Earth Elements
The rare-earth elements (REE) or lanthanoids (at times wrongly referred to as lanthanides) consist of 15 elements plus scandium and yttrium, which are not part of the group, but are included as REE, because they often occur in the same ore body. Therefore,
in total there are17 REE. They are all very similar, almost indistinguishable, silvery colour and exhibit very similar chemical properties.
The principal sources of rare-earth elements are the minerals bastnäsite, monazite, and loparite and the lateritic ion-adsorption clays. Coal and coal by-products are a potential source of critical elements including REE with estimated amounts in the range of 50 million metric tons, in North America alone.
Despite their name, rare-earth elements are relatively plentiful. For example, cerium is the 25th most abundant element in earth’s crust, more common than copper. However, since they cannot be found in clusters that could be mined easily, they are considered quite rare. Some, such as Promethium, are radioactive and are generated as a result of decaying uranium 238, usually mixed with Thorium. Most REE are created by supernova nucleosynthesis.
China produces about 85% of the world’s REE, mostly as a by-product of iron mining operations in Inner Mongolia. Almost 95% of heavy REE are produced by China, including 100% of dysprosium. Currently worldwide demand for rare earth elements is exceeding their supply by 70,000 tons annually. This is a major strategic /industrial concern for North American and European countries as uses, applications, and demand for rare-earth elements keeps expanding quite rapidly.
REE mines are often in countries where environmental and social standards are very low, causing human rights violations, deforestation and contamination of land and water. Mining, refining, and recycling of rare earths have serious environmental consequences if not properly managed. Low-level radioactive tailings present serious hazards and improper handling of these substances can result in extensive environmental damage.
Advances in the recycling technology have made extraction of rare earths from various manufactured materials more feasible. It is estimated by the USGS that about 300,000 tons of REE remain stored in used electronics, magnets, and batteries. France and Japan are the most advanced countries in recycling of REE.
Over the past 15 years, research and our knowledge have evolved regarding the characteristics and uses of rare earth elements such that many of these materials have become indispensable in the manufacture of advanced technological equipment with a particular focus on clean-energy applications such as wind turbines and hybrid/electric cars. Most consumer electronic devices require REE and new technologies being developed for water purification, desalination, magnetic refrigeration, and more energy-efficient
light bulbs will need REE as well. Canada currently relies upon the import of REE-containing components for the manufacture of key products in many industries. Thus, while the sector directly involved in REE is worth roughly $2 billion (130,000 tpa), its indirect value throughout the global economy may be measured in trillions of dollars.
Rare earth elements are difficult to separate from one another once they have been liberated from REE-bearing minerals. At the atomic level, the lanthanide elements have a similar outer electronic structure, with this structure shielding the so-called 4f electrons within the atom. The presence and incremental filling of these electron orbitals are key characteristics of the lanthanides. This unique structure leads to REE ions that are very similar in size to each other. It is this similarity in ionic radii across the group, not the adjacency of their atomic numbers, which gives rise to the similar chemical properties associated with each of the REEs. This makes them difficult to separate, even with the use of intensive processes such
as solvent extraction (SX). CVMR has a proprietary process for recycling of REE.